Qelbree and
ADHD FAQs
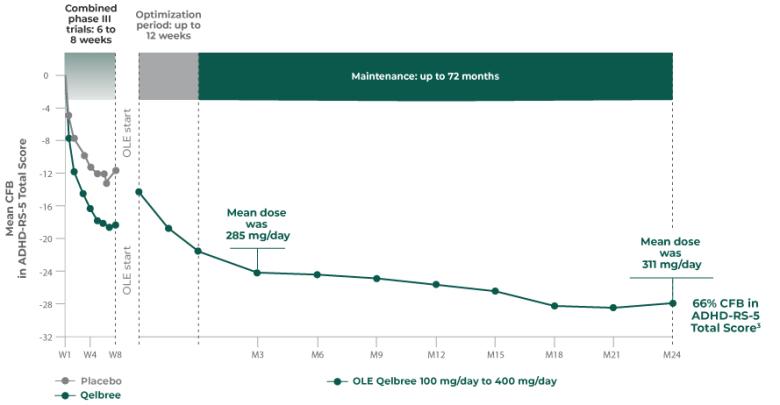
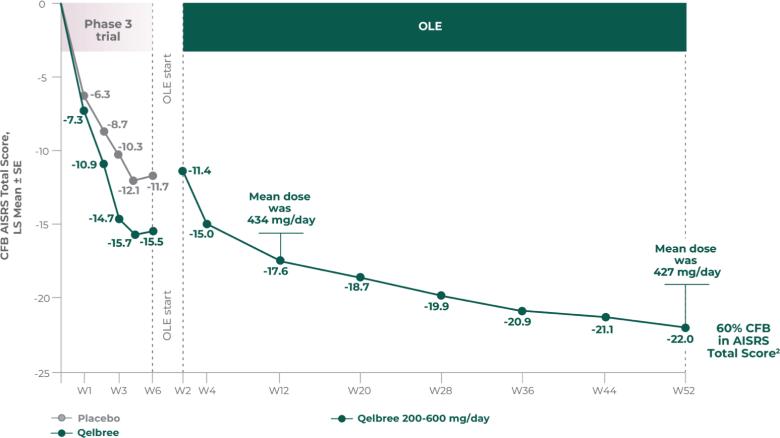
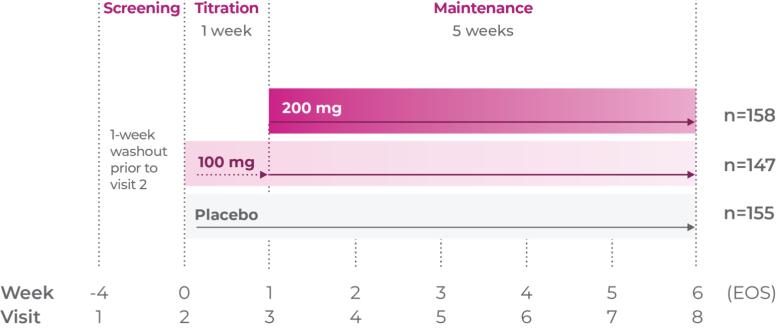
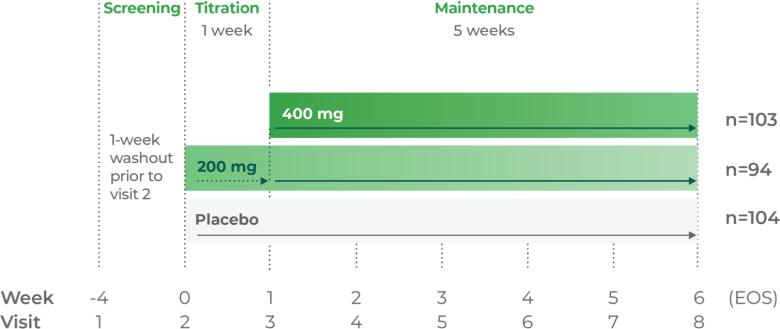
Qelbree is a once-daily sprinkleable capsule that treats ADHD in patients ages 6 and older.1
The most common adverse reactions for children and adolescents (occurring at ≥5% and at least twice the placebo rate for any dose) were somnolence, decreased appetite, fatigue, nausea, vomiting, insomnia, and irritability. And in adults, insomnia, headache, somnolence, fatigue, nausea, decreased appetite, dry mouth, and constipation.1,2
Ask your patients about their medical conditions, including if they:
- Have, or their family has, a history of suicide, bipolar disorder, depression, mania, or hypomania
- Have blood pressure or heart rate problems
- Have severe kidney disease or dysfunction. Their dose of Qelbree and/or other medicines may need to be reduced3
- Are pregnant or plan on becoming pregnant
- Are breastfeeding or plan to breastfeed
Ask your patients about all the medicines they take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking Qelbree with certain other medicines may cause side effects or affect how well they work.
Do not administer Qelbree if patients also take a monoamine oxidase inhibitor (MAOI) or within 14 days of discontinuation of an MAOI (e.g., isocarboxazid, phenelzine, selegiline, tranylcypromine) or take medications that are sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range (e.g., alosetron, duloxetine, ramelteon, tasimelteon, tizanidine, theophylline).
Do not administer Qelbree if patients are allergic to any of the ingredients in Qelbree. You can direct your patients to the Medication Guide for a complete list of ingredients in Qelbree.
Qelbree is the only ADHD treatment with serotonin (5-HT2c) pharmacodynamics approved in the FDA product labeling.1,4
- As outlined in section 12.2, viloxazine binds to and inhibits the norepinephrine transporter (Ki=0.13 μM)
- In addition, viloxazine binds to and exhibits partial agonist activity at the serotonin 5-HT2C receptor (Ki=0.66 μM)
- Qelbree is not a controlled substance
In a study involving 15 healthy lactating women, it was shown that the transfer of viloxazine into breast milk is low. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for Qelbree and any potential adverse effects on the breastfed child from Qelbree or from the underlying maternal condition.1
Consider the available data when discussing treatment options with your patients, and to make individualized decisions based on each patient’s unique circumstances.
Store Qelbree capsules at room temperature 68-77°F (excursions permitted between 59-86°F) and 20-25°C (excursions permitted between 15-30°C). Keep Qelbree and all medicines out of reach of children.1
Once-daily Qelbree shows no evidence of abuse potential or dependence – minimizing the risk of misuse and diversion.2
Qelbree can be taken with or without food, whole, or the entire contents can be sprinkled over a spoonful (teaspoon or tablespoon) of soft food (pudding or applesauce). Consume the entire pudding mixture within 15 minutes or the applesauce mixture within 2 hours, without chewing. Do not store for future use. Capsules and their contents should not be cut, crushed, or chewed.1,2

100 mg: yellow, opaque body and cap (printed “SPN” on the cap, “100” on the body)1

150 mg: lavender, opaque body and cap (printed “SPN” on the cap, “150” on the body)1

200 mg: light green, opaque body and cap (printed “SPN” on the cap, “200” on the body)1
You might also be interested in: