Help your ADHD
patients make the
Qelbree switch1
Qelbree is today's fastest-growing ADHD non-stimulant brand2*
*IQVIA non-stimulant market data 5/2024-5/2025

Is your ADHD patient still:
- feeling overwhelmed?3
- being chronically disorganized?3
- exhibiting poor time management/chronic lateness?3
- experiencing low self-esteem?4
- struggling to manage daily activities/chores?3,4
- finding making/keeping friendships difficult?4
- lacking in self-motivation or self-management?3,4
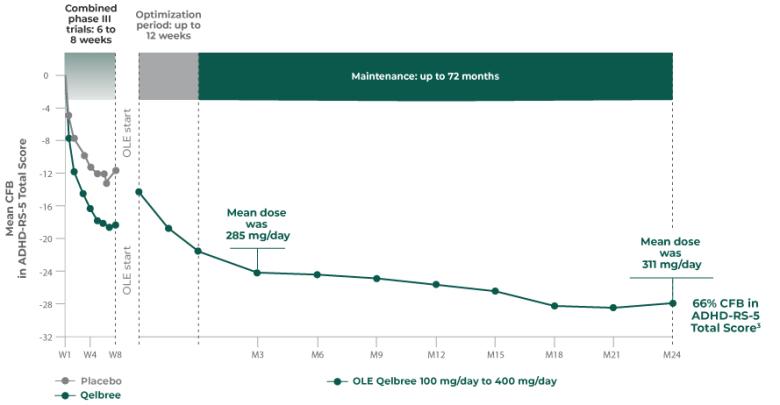
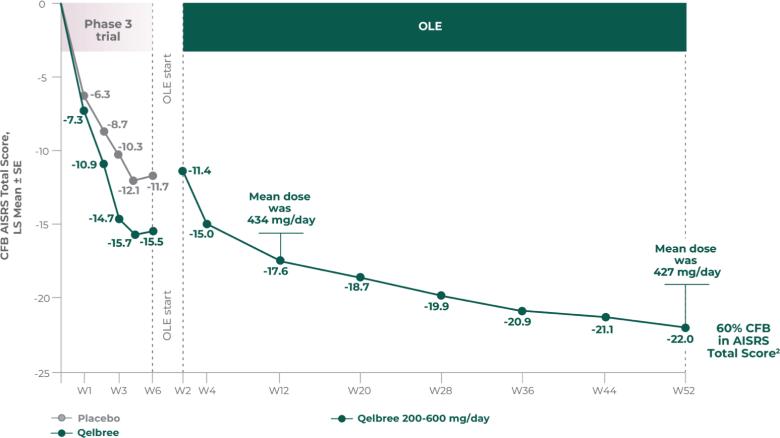
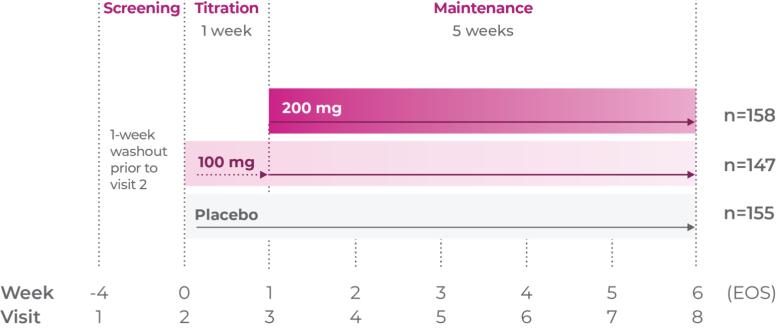
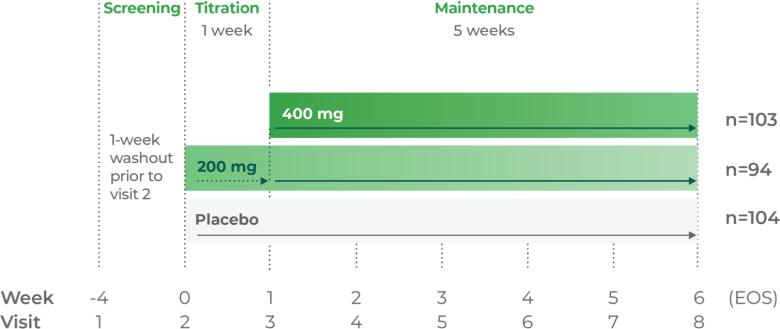
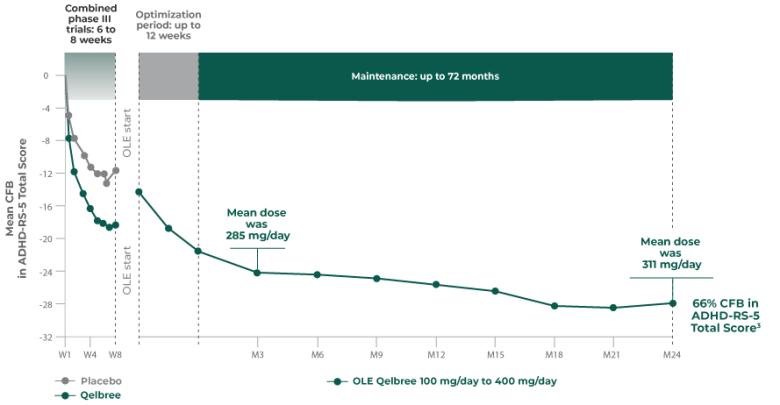
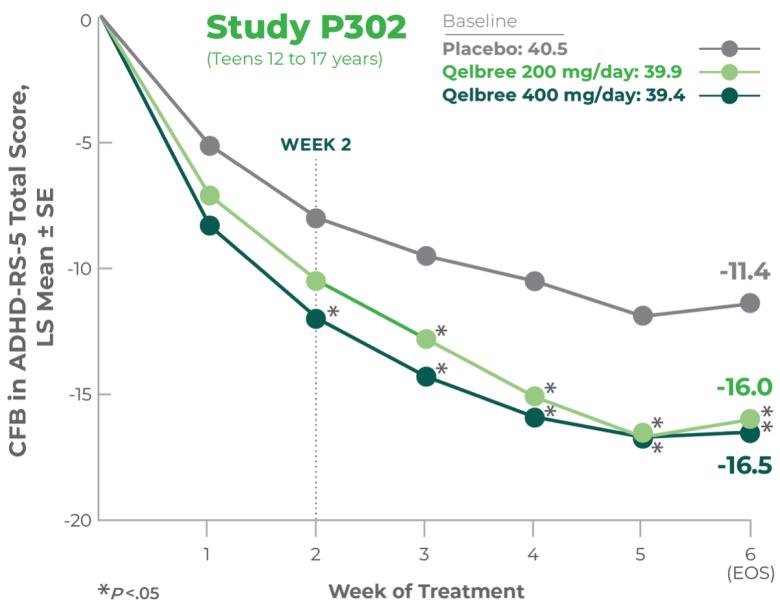
Consider Qelbree—a non-stimulant that works1

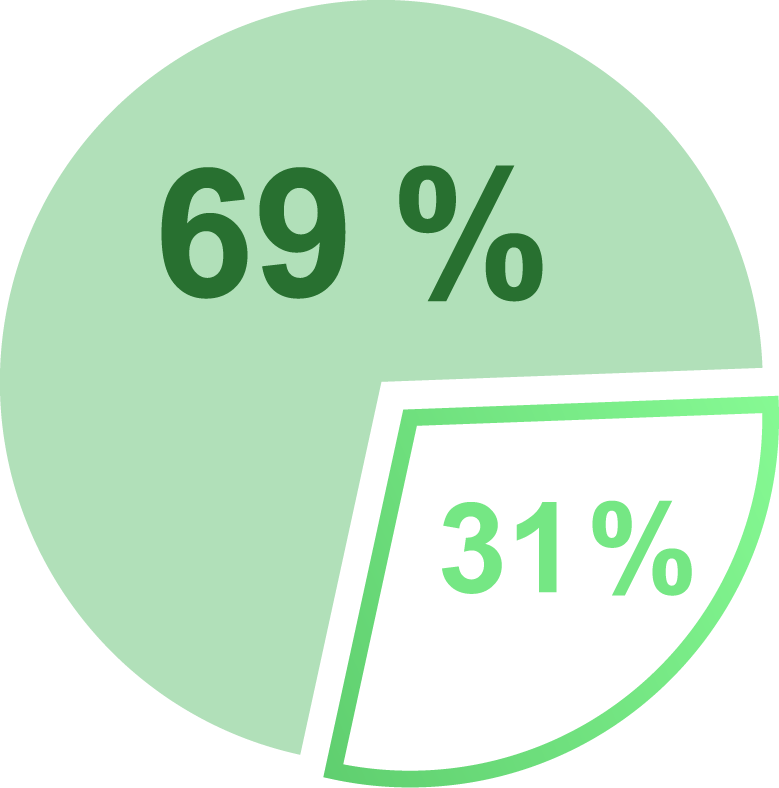
National prescription data† shows that 69% of patients were prescribed Qelbree because a change in their previous ADHD treatment was needed, and 31% of patients taking Qelbree were new to prescription ADHD treatment2
Market data† shows that healthcare providers like you are switching patients like Ava from stimulant ADHD medications to Qelbree2
49% of patients who were switched to Qelbree came from prior ADHD treatment with one of these stimulants2:
- Vyvanse®
- AMP ER
- MPH ER
- MPH IR
- AMP IR
- DEXMPH/Focalin®
- Other
Border
And it shows that they’re switching patients like Christine from other non-stimulant ADHD medications to Qelbree2
51% of patients who switched to Qelbree came from prior ADHD treatment with one of these non-stimulants2:
- Atomoxetine/Strattera®
- Guanfacine/Intuniv®
- Other
Border
Patients like Alex, who are new to prescription ADHD treatment, are also being prescribed Qelbree, according to the market data2†
†Source: IQVIA Xponent market dynamics data, R52W, as of April 4, 2025.
N=108,735 prescriptions
What your peers are saying about Qelbree
Days can be chaotic for many adult patients with ADHD. Qelbree offers full-day coverage and is proving to be a good option for many of my patients
– Greg Mattingly, MD
I have developed confidence that Qelbree can help my ADHD patients to reach their treatment goals
– Andrew Cutler, MD
Qelbree has made me feel confident I have a non-stimulant I can work with for many of my adult patients with ADHD
– Rakesh Jain, MD
You might also be interested in: