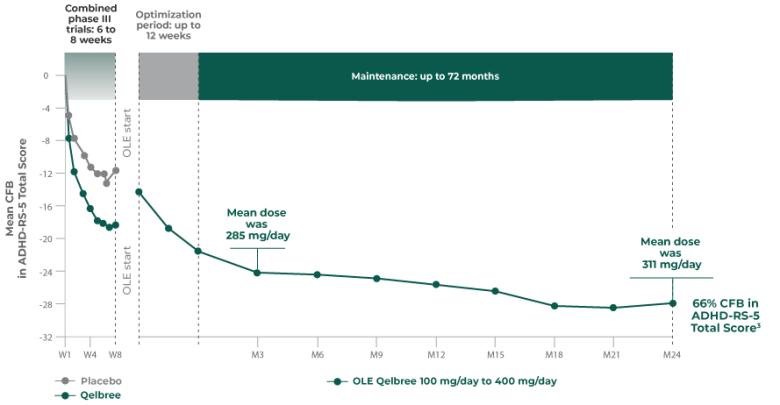
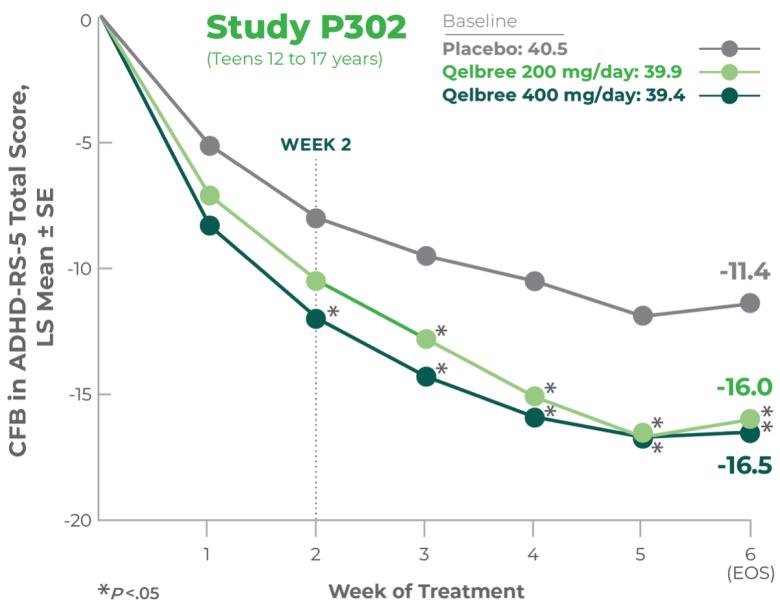
INDICATION
Qelbree is indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in adult and pediatric patients 6 years and older.
IMPORTANT SAFETY INFORMATION
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
In clinical studies, higher rates of suicidal thoughts and behaviors were reported in [read more] patients with ADHD treated with Qelbree than in patients treated with placebo. Closely monitor all Qelbree-treated patients for clinical worsening and for emergence of suicidal thoughts and behaviors.
In clinical studies, higher rates of suicidal thoughts and behaviors were reported in patients with ADHD treated with Qelbree than in patients treated with placebo. Closely monitor all [read more] Qelbree-treated patients for clinical worsening and for emergence of suicidal thoughts and behaviors.
CONTRAINDICATIONS
- Concomitant administration of a monoamine oxidase inhibitor (MAOI), or dosing within 14 days after discontinuing an MAOI, because of an increased risk of hypertensive crisis
- Concomitant administration of sensitive CYP1A2 substrates or CYP1A2 substrates with a narrow therapeutic range
WARNINGS & PRECAUTIONS
- Suicidal thoughts and behaviors: Closely monitor all Qelbree-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes
- Heart rate, blood pressure increases: Qelbree can cause an increase in diastolic blood pressure and heart rate. Assess these measures prior to starting therapy, following increases in dosage, and periodically during therapy
- Activation of mania or hypomania: Noradrenergic drugs may induce a manic or mixed episode in patients with bipolar disorder. Prior to initiating treatment with Qelbree, screen patients to determine if they are at risk for bipolar disorder. Screening should include a detailed psychiatric history, including a personal or family history of suicide, bipolar disorder, and depression
- Somnolence and fatigue: Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, due to potential somnolence (including sedation or lethargy) and fatigue, until they know how they will be affected by Qelbree
ADVERSE REACTIONS
The most common adverse reactions (≥ 5% and at least twice the rate of placebo for any dose) in patients 6 to 17 years were somnolence, decreased appetite, fatigue, nausea, vomiting, insomnia, and irritability, and in adults, insomnia, headache, somnolence, fatigue, nausea, decreased appetite, dry mouth, and constipation.
PREGNANCY
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Qelbree during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or by visiting www.womensmentalhealth.org/preg.
Please see full Prescribing Information, including Boxed Warning.