Convenient,
once-daily dosing1,2
Non-stimulant Qelbree is prescribed once-daily (AM or PM) for full 24-hour exposure.1,2
Titrate weekly as needed to optimize ADHD symptom control1,2
Children 6 to 11 years1
Titrate Qelbree 100 mg/week over 1 to 3 weeks as needed to reach effective dose1
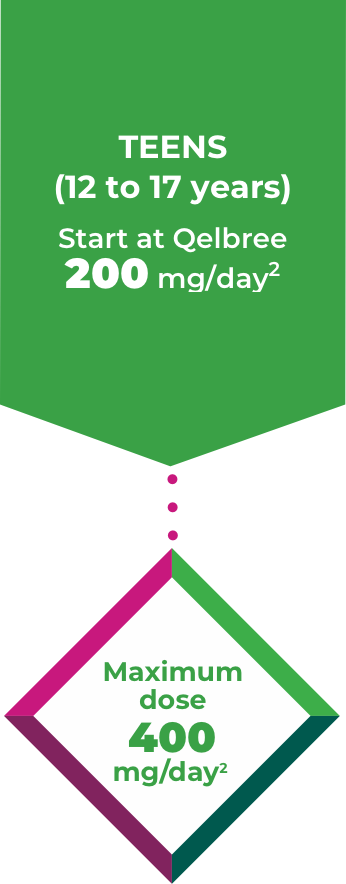
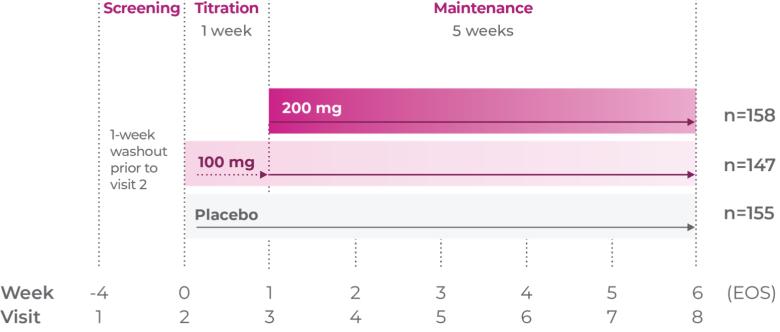
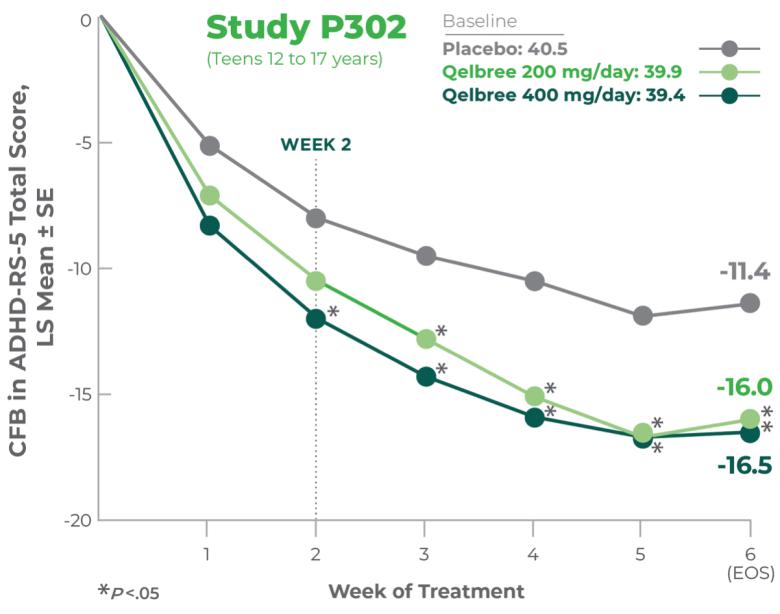
Teens 12 to 17 years1
Titrate Qelbree 200mg/week over 1 week as needed to reach an effective dose1
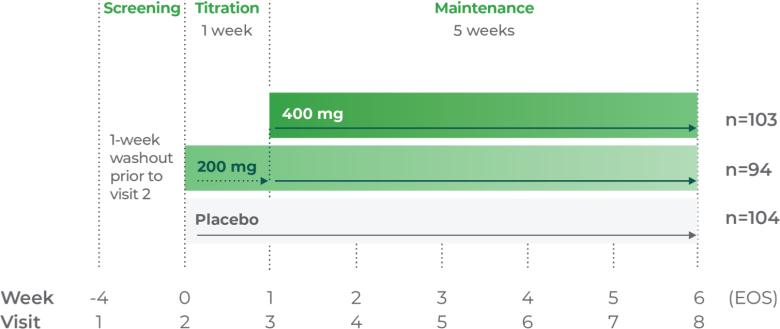
Adults 18 years and older1
Titrate Qelbree 200 mg/week over 1-2 weeks as needed to reach an effective dose1
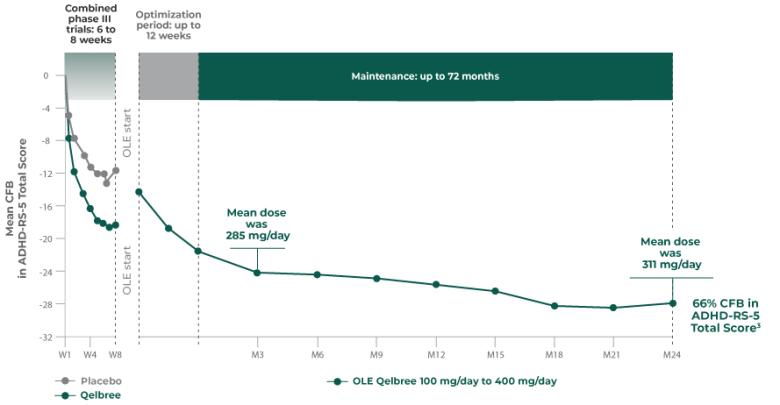
Adult phase III (Study P306) Mean dose at EOS (6 weeks) was 504 mg/day (n=354)2
Abbreviations: EOS, end of study.
Dosing safety information1
- Severe renal impairment: Initiate Qelbree at 100 mg and increase by 50 mg to 100 mg at weekly intervals to a maximum recommended dosage of 200 mg once daily
3 strengths allow for flexible dosing1
Straightforward, easy administration1
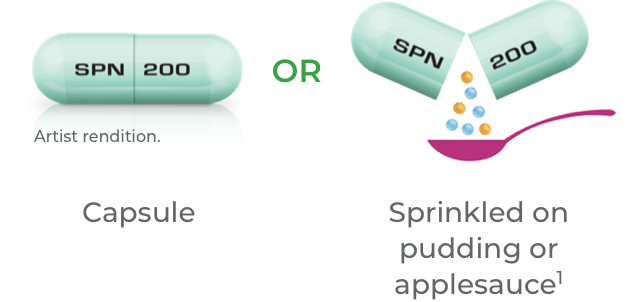
Capsule can be taken whole or entire contents can be sprinkled over a spoonful (teaspoon or tablespoon) of soft food (pudding or applesauce). Consume the entire contents of the pudding mixture within 15 minutes or applesauce mixture within 2 hours, without chewing. Do not store for future use.1,2
- Capsules and their contents should not be cut, crushed, or chewed
- Can be taken with or without food
- Dose will depend on response to medication
Qelbree capsules are available in 3 dosage strengths: 100 mg capsules, 150 mg capsules, and 200 mg capsules.1
Capsules shown are not actual size.

Refills without the need for a new prescription every month
Qelbree, a non-stimulant therapy, offers ADHD multi-symptom control.1,2
You may even be able to prescribe a 90-day supply for more convenience.*
*Subject to any plan restrictions.
You might also be interested in: